Abstract
Introduction
The phase 3 ECHELON-2 study (NCT01777152) compared frontline treatment with brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisone (A+CHP) to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) for patients (pts) with systemic anaplastic large cell lymphoma (sALCL) or other CD30+ peripheral T-cell lymphomas (PTCL). At 5 years, A+CHP continues to provide clinically meaningful improvement in progression-free survival (PFS) (hazard ratio [HR] 0.70 [95% confidence interval {CI}: 0.53, 0.91], P=0.0077) and overall survival (OS) (HR 0.72 [95% CI: 0.53, 0.99], P=0.0424) vs CHOP. Ongoing remission was observed in ~60% of pts with sALCL, with a manageable safety profile, including continued resolution or improvement of peripheral neuropathy (Horwitz S, et al. ASH 2020). Due to the rarity of this disease and lack of data on prospective, uniformly treated pts, ECHELON-2 provides a large and unique prospective data set with potential utility in informing future studies of PTCL. Herein, we report a series of exploratory analyses in prespecified subgroups based on age, gender, and histology to supplement the data from the full 5-year analysis.
Methods
ECHELON-2 (N=452) is a randomized, double-blind, double-dummy, placebo-controlled, multicenter study. Eligible pts with previously untreated CD30+ PTCL were randomized 1:1 to A+CHP or CHOP for 6 or 8 cycles. Randomization was stratified by histological subtype and international prognostic index (IPI) score. The primary endpoint of PFS was assessed per blinded independent central review in the primary analysis and per investigator in this exploratory subgroup analysis. Outcomes by age <60 and ≥60 years, gender, and PTCL subtype (PTCL-not otherwise specified [NOS], angioimmunoblastic T-cell lymphoma [AITL], and sALCL by IPI categories) were assessed. ECHELON-2 was not powered or designed to compare efficacy of therapy within individual histologic subtypes other than sALCL or other baseline characteristics.
Results
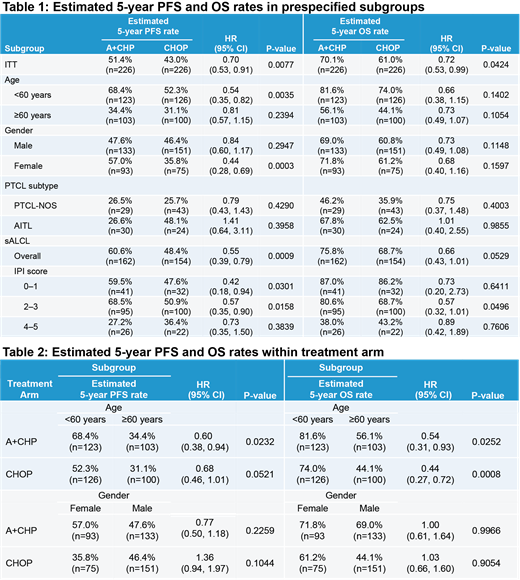
Among all pts <60, the median PFS was not reached versus vs 17.48 months among all pts ≥60. When assessing by treatment, PFS favored A+CHP vs CHOP for both age groups (Table 1). PFS outcomes in the <60 subgroup were superior vs the ≥60 subgroup in both arms (Table 2). In addition, the median OS for those <60 was not reached vs 60.4 months for those ≥60. OS was also longer in those treated with A+CHP regardless of age (<60, HR 0.66 [95% CI: 0.38, 1.15], P=0.1402; ≥60, HR 0.73 [95% CI: 0.49, 1.07], P=0.1054).Within each arm, OS was superior in the <60 vs ≥60 age group (A+CHP, HR 0.54 [95% CI: 0.31, 0.93], P=0.0252; CHOP, HR 0.44 [95% CI: 0.27, 0.72], P=0.0008).
The potential association of gender with PFS outcomes varied within each treatment arm: males on the CHOP arm had higher PFS than females while females had higher PFS than males on the A+CHP arm (Table 2). In addition, the OS benefit of A+CHP vs CHOP was consistent for both genders (female, HR 0.68 [95% CI: 0.40, 1.16], P=0.1597; male, HR 0.73 [95% CI: 0.49, 1.08], P=0.1148). Females and males had similar OS within each arm (A+CHP, female vs male: HR 1.00 [95% CI: 0.61, 1.64], P=0.9966; CHOP, female vs male: HR 1.03 [95% CI: 0.66, 1.60], P=0. 9054).
Among all pts with PTCL-NOS, the median PFS and OS were 13.57 months and 46.4 months. When assessing by treatment arm, the PFS (Table 1) and OS (HR 0.75 [95% CI: 0.37, 1.48], P=0.4003) HRs were similar to the intent-to-treat (ITT) population. Among all pts with AITL, the median PFS was 23.75 months and the median OS was not reached. When assessing by treatment arm, the PFS (Table 1) favored CHOP and OS was similar (HR 1.01 [95% CI: 0.40, 2.55], P=0.9855).
In the sALCL subgroup, PFS was superior in those treated with A+CHP overall and across IPI subgroups: 0-1, 2-3, 4-5 (Table 1). OS was also longer in the A+CHP arm (HR 0.66 [95% CI: 0.43, 1.01], P=0.0529) overall and across IPI subgroups: 0-1 (HR 0.73 [95% CI: 0.20, 2.73], P=0.6411), 2-3 (HR 0.57 [95% CI: 0.32, 1.01], P=0.0496), and 4-5 (HR 0.89 [95% CI: 0.42, 1.89], P=0.7606).
Conclusions
ECHELON-2 results at 5 years demonstrate a generally consistent benefit of A+CHP over CHOP across subgroups and for the ITT population. Furthermore, this large prospective dataset of pts with PTCL has redefined efficacy outcomes for this population and provides important benchmark data to inform future studies.
Horwitz: Affimed: Research Funding; Acrotech Biopharma, Affimed, ADC Therapeutics, Astex, Merck, Portola Pharma, C4 Therapeutics, Celgene, Janssen, Kura Oncology, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, Shoreline Biosciences, Inc, Takeda, Trillium Th: Consultancy; ADC Therapeutics, Affimed, Aileron, Celgene, Daiichi Sankyo, Forty Seven, Inc., Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio.: Consultancy, Research Funding; Aileron: Research Funding; Celgene: Research Funding; C4 Therapeutics: Consultancy; Crispr Therapeutics: Research Funding; Daiichi Sankyo: Research Funding; Forty Seven, Inc.: Research Funding; Kura Oncology: Consultancy; Kyowa Hakko Kirin: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Myeloid Therapeutics: Consultancy; ONO Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy, Research Funding; Secura Bio: Consultancy; Shoreline Biosciences, Inc.: Consultancy; Takeda: Consultancy; Trillium Therapeutics: Consultancy, Research Funding; Tubulis: Consultancy; Verastem/Securabio: Research Funding. Savage: Astra-Zeneca: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Takeda: Other: Institutional clinical trial funding; AbbVie: Consultancy, Honoraria; Merck: Consultancy, Honoraria, Other: Institutional clinical trial funding; BMS: Consultancy, Honoraria, Other: Institutional clinical trial funding; Seattle Genetics: Consultancy, Honoraria; Roche: Research Funding; Beigene: Other: Institutional clinical trial funding; Genentech: Research Funding. Illidge: Seagen Inc.: Research Funding. Advani: Astellas/Agensys: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Bristol Myer Squibb: Membership on an entity's Board of Directors or advisory committees; Cell Medica: Membership on an entity's Board of Directors or advisory committees; Forty Seven: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genetech Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceutical: Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Kura: Research Funding; Kyowa: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Millenium: Research Funding; Pharmacyclics: Consultancy, Research Funding; Portola Pharmaceuticals: Consultancy; Regeneron: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Shustov: Seagen Inc.: Research Funding. Bartlett: Pharmacyclics: Research Funding; Millennium: Research Funding; Merck: Research Funding; Kite, a Gilead Company: Research Funding; Janssen: Research Funding; Genentech: Research Funding; Forty Seven: Research Funding; Celgene: Research Funding; Bristol Myers Squibb: Research Funding; Autolus: Research Funding; Seagen: Consultancy, Research Funding; Roche/Genentech: Consultancy; ADC Therapeutics: Consultancy, Research Funding. Jacobsen: Takeda: Consultancy; Syros: Consultancy; Janssen: Research Funding; Novartis: Research Funding; Pharmacyclics: Research Funding; Acerta: Research Funding. Koch: Seagen Inc.: Research Funding. Eva: Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Fanale: Seagen, Inc: Current Employment, Current equity holder in publicly-traded company. Fenton: Seagen Inc.: Current Employment, Current equity holder in publicly-traded company. Campana: Seagen Inc.: Research Funding. Dong: Seagen Inc.: Research Funding. Truemper: Seagen Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal